Conjugated System Organic Chemistry
Conjugated system organic chemistry. Generally youll need 3 or more orbitals to classify a molecule as conjugated. Conjugation means alternate position. Organic semiconductors are mainly π-conjugated systems which are classified into two groups based on the weight namely π-conjugated polymers and small molecules.
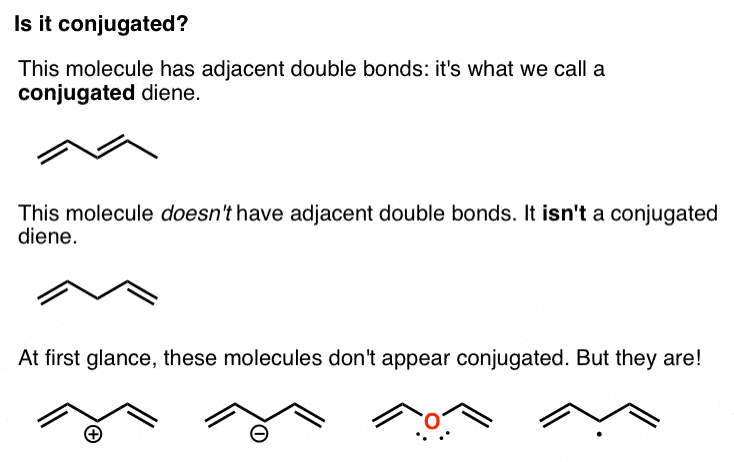
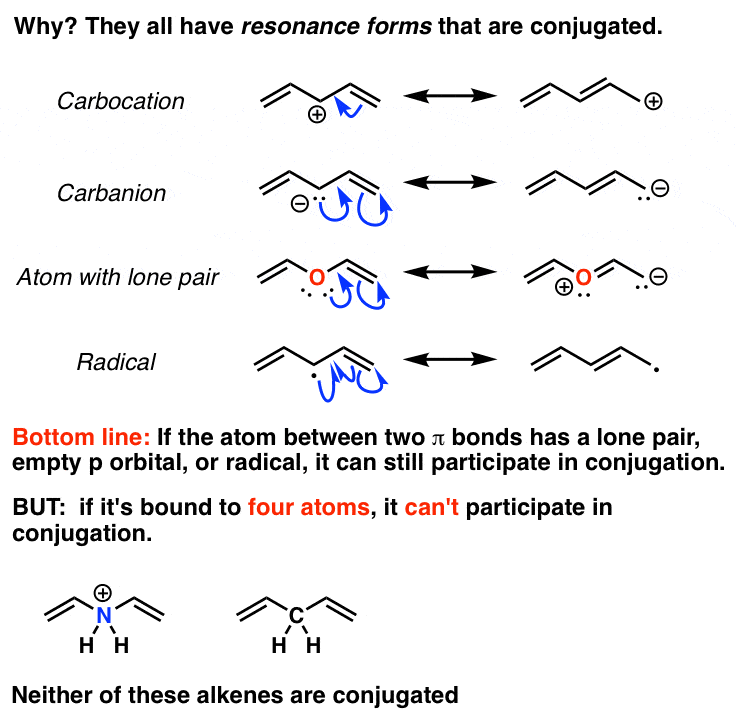
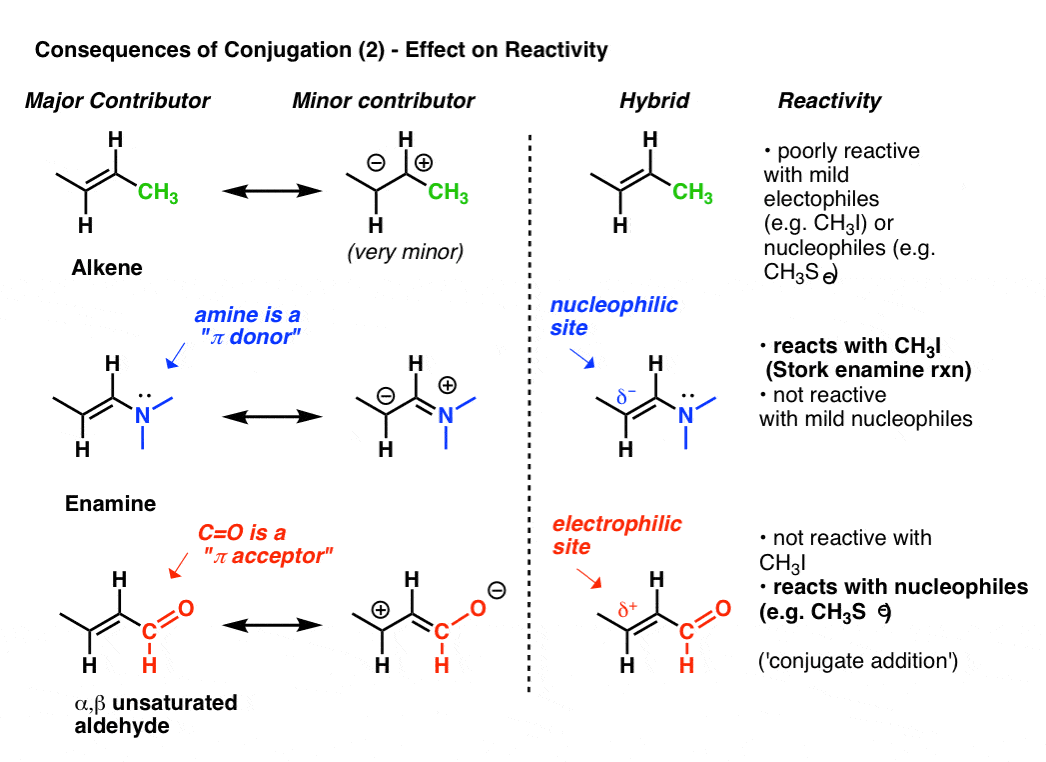
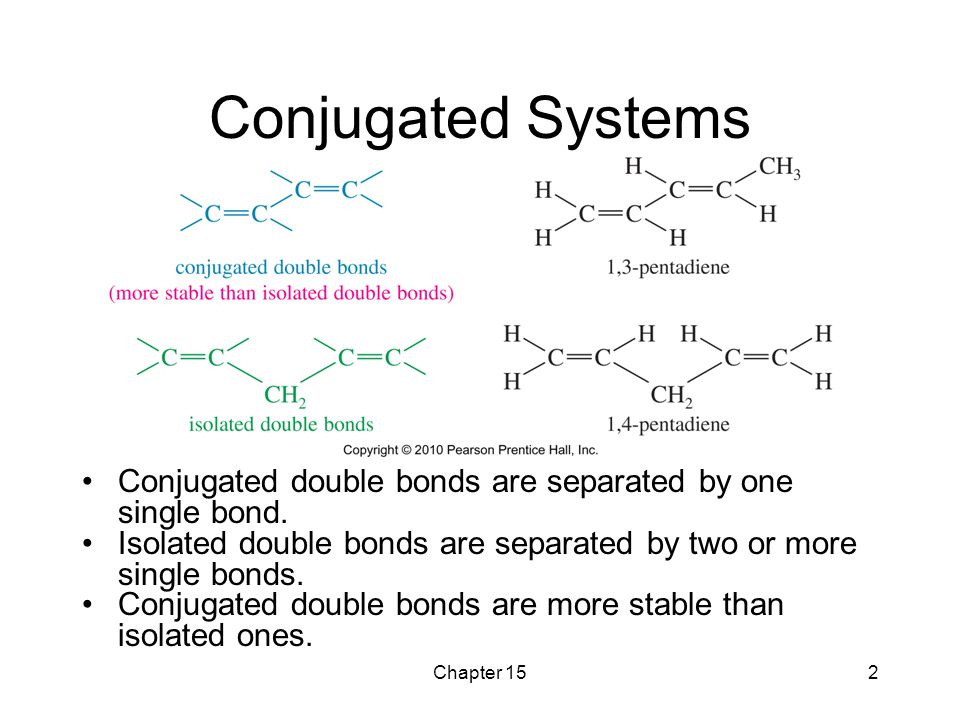
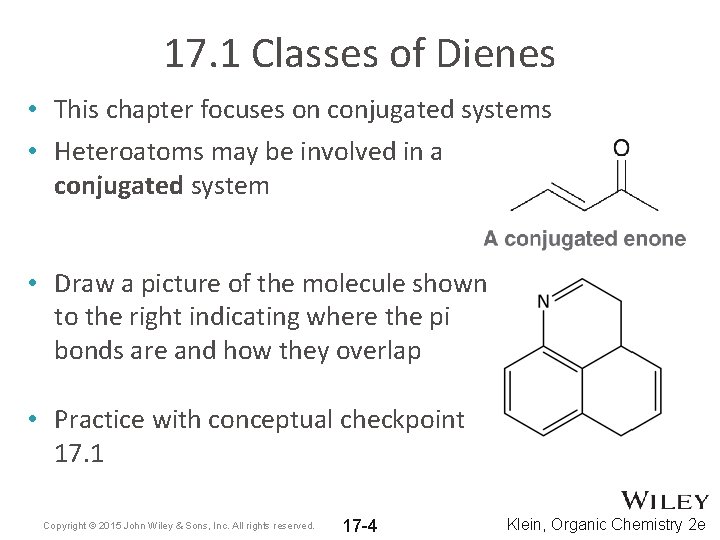
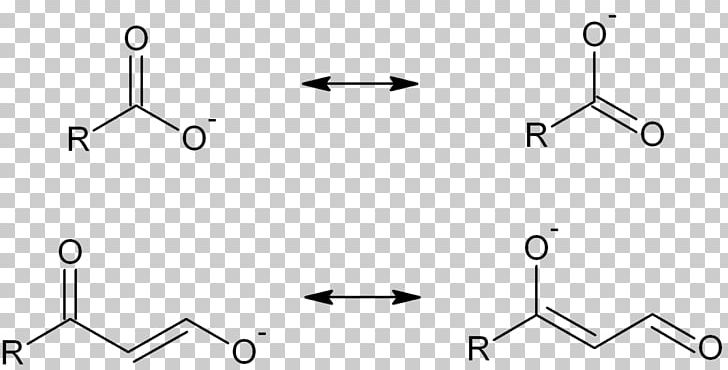
13-1 -- Three Classes of Dienes. - Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond - The p orbital can come from another double or triple bond - The p orbital may be the empty p orbital of a carbocation or a p orbital with a single electron in it a radical - Conjugation affords special stability to the molecule. In chemistry a conjugated system is a system of continuous and parallel p-orbitals with delocalized electrons.
In organic chemistry terms it is used to describe the situation that occurs when π systems egdouble bonds are linked together. Organic compounds are almost endless in number and range in size from small molecules to macromolecules. If pi bonds are in alternate position then it is pi-pi conjugation if pi bond is in alternate position with positive charge Ie.
Why not take an online Organic Chemistry course. Conjugated Systems and UV Spectroscopy - Section 13 of Organic Chemistry Notes is 19 pages in length page 13-1 through page 13-19 and covers ALL youll need to know on the following lecturebook topics. The compound may be cyclic acyclic linear.
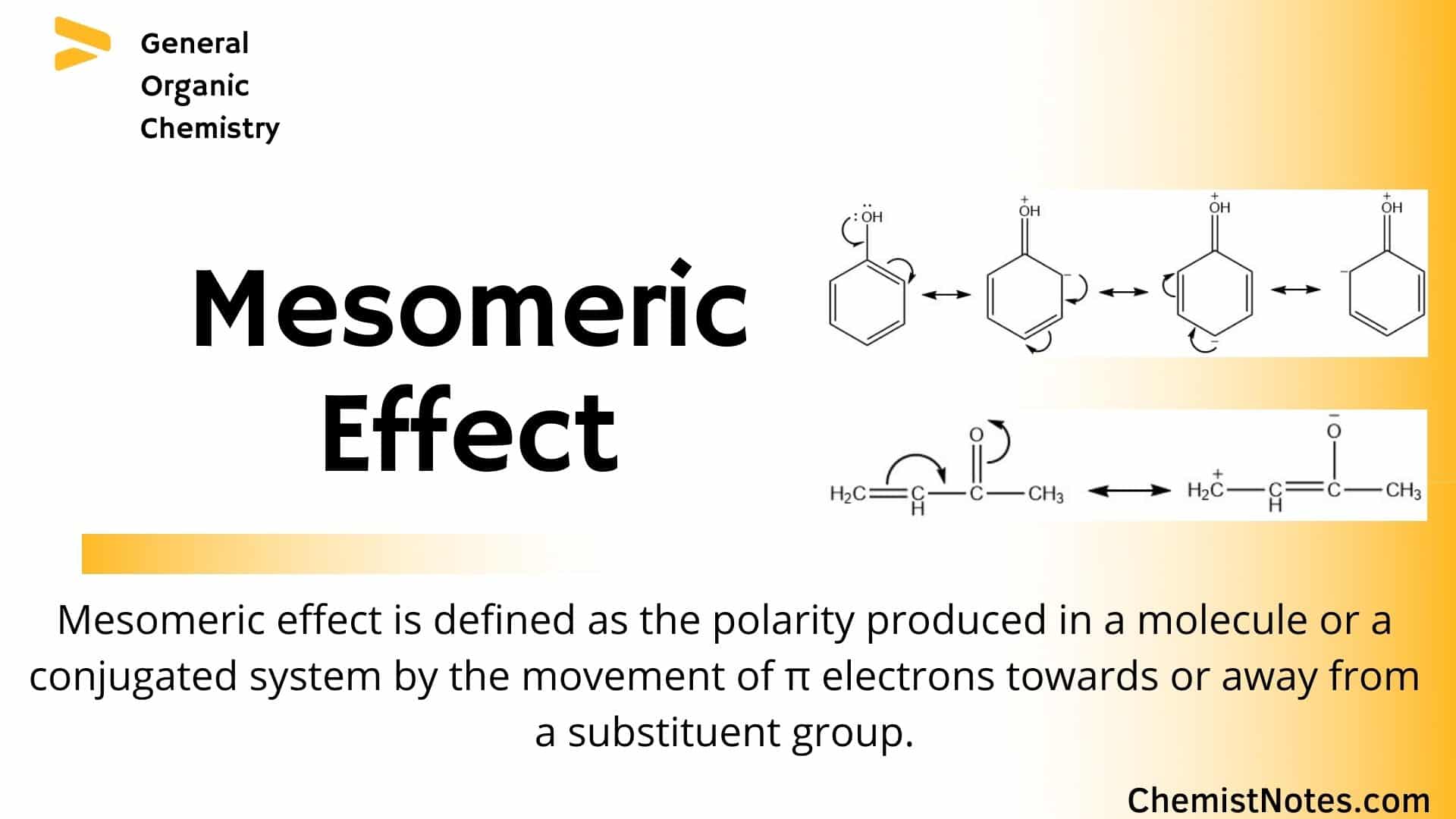
Molecular Orbital Theory explanation of the conjugated polyenes. Vacant orbital then it is pi-positive charge conjuga. Conjugated system in a covalent chemical compound a group or chain of atoms bearing valence electrons that are not engaged in single-bond formation and that modify the behaviour of each otherIf for example a carbonyl group C O and a hydroxyl group OH are widely separated in a molecule each has distinctive properties but in combination they form.
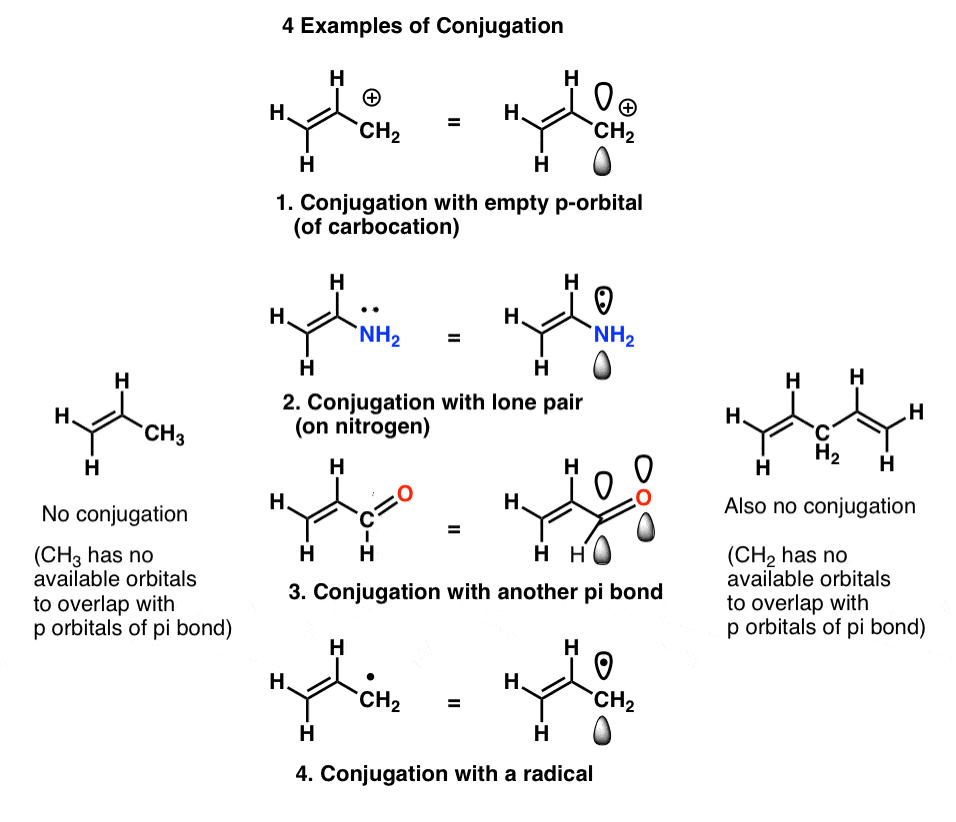
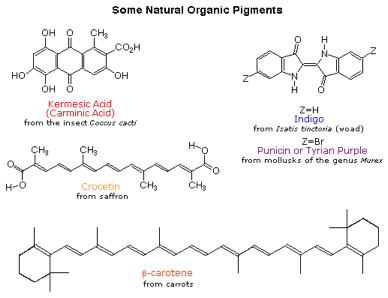
Organic Chemistry Introduction to Conjugated Systems A conjugated system is a type of a molecule where you have multiple p-orbitals interacting with each other. Thus most dyes and most colored compounds occurring in living organisms turn out to be large molecules. In order for an organic molecule to absorb in the visible region of the spectrum it must usually contain very delocalized pi electrons.
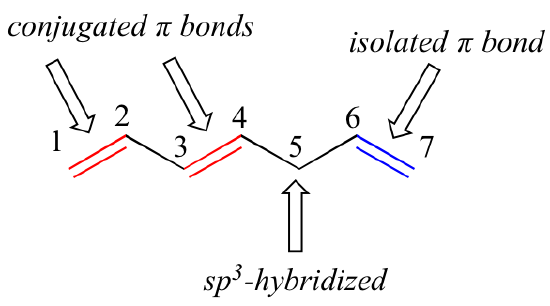
What are conjugated compounds in organic chemistry. An isolated π pi system exists only between a single pair of adjacent atoms egCC.
13-1 -- Three Classes of Dienes.
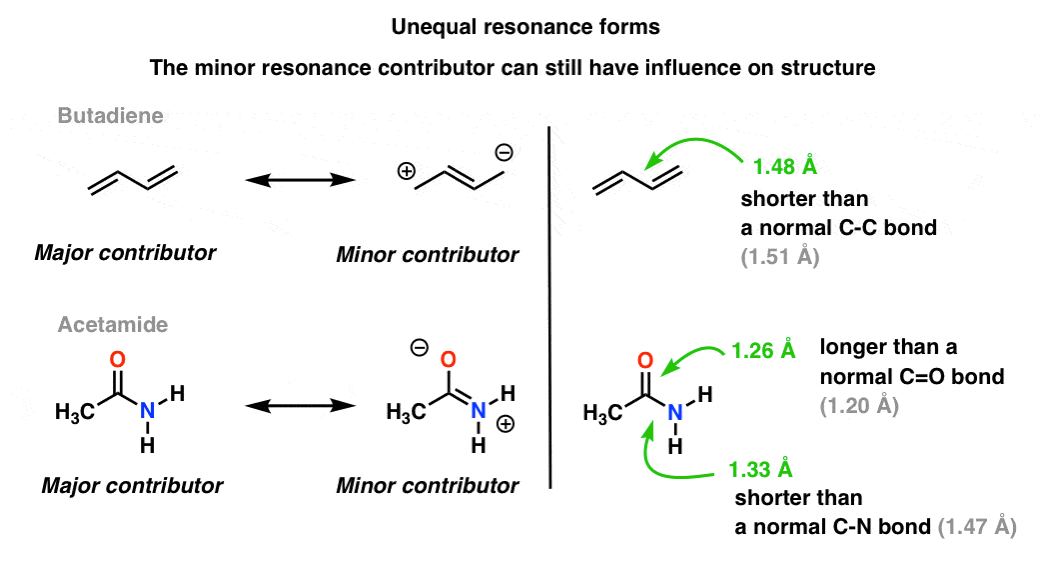
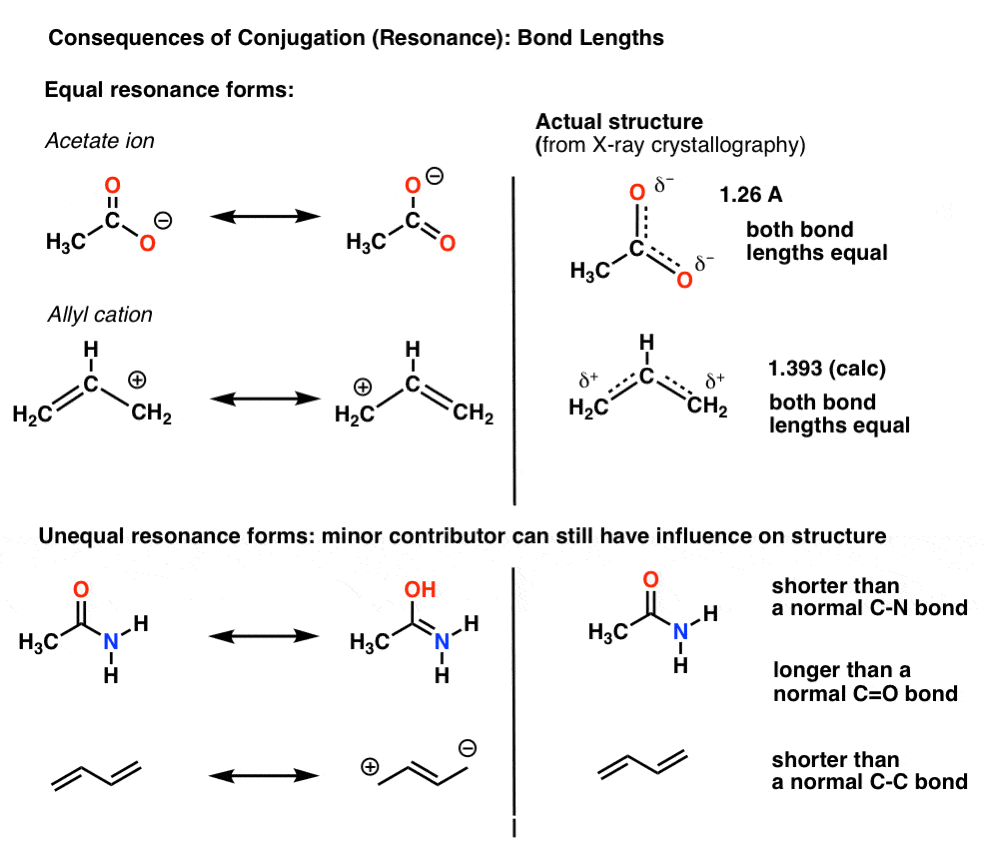
The delocalization of electrons in a molecule is called conjugation in organic chemistry. The delocalization of electrons in a molecule is called conjugation in organic chemistry. One of the most challenging concepts in conjugated system reactions is molecular orbital interactions or MO theory. If pi bonds are in alternate position then it is pi-pi conjugation if pi bond is in alternate position with positive charge Ie. Why not take an online Organic Chemistry course. This delocalisation process of electrons leads to the shortenings or elongations of chemical bonds but at the same time it causes changes in the chemical properties in conjugated molecules as compared to the non-conjugated ones. Organic Chemistry Introduction to Conjugated Systems A conjugated system is a type of a molecule where you have multiple p-orbitals interacting with each other. This allows for its placement at any point needed. The topics addressed are types of unsaturated systems preparations of conjugated systems as well as reactions the impact of conditions and the molecular orbital picture.
The delocalization of electrons in a molecule is called conjugation in organic chemistry. 13-1 -- Three Classes of Dienes. The compound may be cyclic acyclic linear. Molecular Orbital Theory explanation of the conjugated polyenes. For example you can observe the movement of pi electrons in carbon dioxide. The delocalization of electrons in a molecule is called conjugation in organic chemistry. - Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond - The p orbital can come from another double or triple bond - The p orbital may be the empty p orbital of a carbocation or a p orbital with a single electron in it a radical - Conjugation affords special stability to the molecule.



























Post a Comment for "Conjugated System Organic Chemistry"